Effects of testosterone treatment on body fat, lean mass, symptoms and leptin resistance in obese men on a calorie-restricted diet
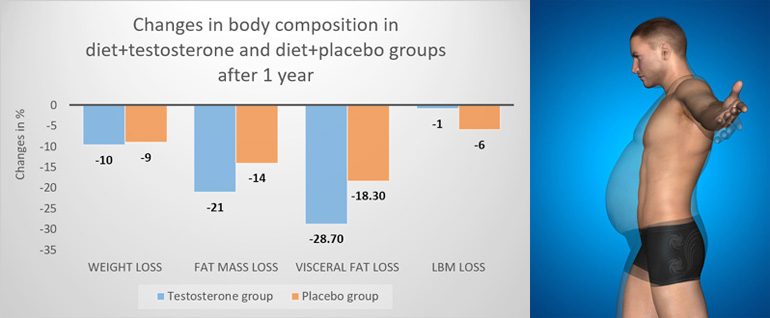
It is well-documented that the relation between testosterone deficiency and body fat is bi-directional; low testosterone levels contribute to the development of excessive body fat accumulation, and an excessive amount of body fat contributes to a reduction in testosterone levels.[1-3]
Here I present a series of three reports from a study that specifically investigated if testosterone therapy has beneficial effects on body composition, symptomatic response, adipokines (hormones secreted by fat cells, such as leptin and adiponectin) and gut hormones, over and above caloric restriction alone.[4-6]
Key Points
- Compared to diet alone, combining diet + testosterone therapy results in a greater reduction in fat mass (-2.9 kg) and visceral fat, and a reduced loss of lean mass after 1 year.
- Dieting men who receive testosterone therapy display higher physical activity levels than dieting men not receiving testosterone therapy.
- The elevation in testosterone levels by diet alone is not enough to optimize body composition results. Diet alone results in less body fat reduction and more lean mass loss than diet + testosterone therapy.
- Diet + testosterone therapy ameliorates symptoms long-term after a diet. Diet alone does not confer long-term symptomatic improvements.
- Diet + testosterone therapy, but not diet alone, reduces leptin resistance.
What is known
Effectiveness of obesity treatments is commonly evaluated based on degree of weight loss.[7-10] However, it is excess body fat that causes most obesity-associated health risks and is associated with increased mortality, independent of body mass index (BMI).[11] Caloric restriction is a foundation in obesity treatment interventions.[8-10] However, the drawbacks with caloric restriction are that adherence to a reduced calorie diet is low, and a large part of the weight loss is composed of lean mass.[12] The loss of lean mass is a major drawback, as a reduced lean mass is an important risk factor for disability, morbidity and mortality.[13] Chronic diseases related to poor lifestyle behaviors account for more than two-thirds of deaths [14], and detrimental alterations in muscle metabolism and loss of lean (muscle) mass play an important role in these disease outcomes.[15] Sub-optimal lean mass can also occur in younger people and has detrimental health consequences regardless of age.[16]
In men, obesity is the single most important factor associated with low testosterone, overriding the effects of age and comorbidities.[1, 2, 17, 18] Obese men have 30% lower total testosterone levels compared to lean men, and 40% have levels lower than 12 nmol/L.[17] On the other hand, low testosterone levels increase body fat accumulation and the risk of obesity.[1] This bidirectional relationship between lowered testosterone and obesity is supported by clinical studies; weight loss increases testosterone proportionally to weight loss [1] and testosterone treatment reduces body fat.[19] However, whether testosterone treatment augments fat loss over that seen with caloric restriction alone, or prevents diet-associated loss of muscle mass, is unknown. It is also not know if testosterone treatment improves symptoms related to testosterone deficiency, over and above the symptomatic improvement after weight loss alone.
What this study adds
100 obese men (BMI>/= 30 kg/m2) with a total testosterone level of or below 12 nmol/L (346 ng/dL) and a median age of 53 years ( range 47-60) receiving 10 weeks of a very low energy diet (640 calories per day and two cups of low-starch vegetables) followed by 46 weeks of weight maintenance were randomly assigned at baseline to 56 weeks of 10-weekly intramuscular testosterone undecanoate (n = 49) or matching placebo (n = 51).[4-6] 82 men completed the study.
Body composition
At study end, compared to controls, testosterone treated men had a significantly greater reduction in fat mass of -2.9 kg and in visceral fat than men in the placebo group. There was a trend toward a larger loss of lean mass in the placebo group of -4.8 kg compared to -3.9 kg in the testosterone group after the initial 10 week diet.
Notably, the testosterone treated men regained 3.3 kg lean mass during weight maintenance, so that at study end (10 weeks diet followed by 46 weeks weight maintenance), they had an attenuated reduction in lean mass compared to non-testosterone treated men, -0.6 vs. 4 kg, respectively. Figure 1 summarises these changes.
Figure 1: Change in body composition outcomes compared to baseline between groups.
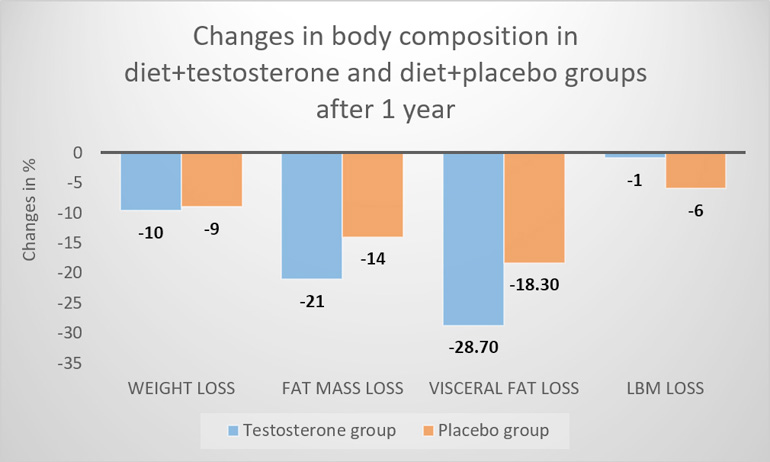
LBM; lean body mass
Data from Ng Tang Fui M, Prendergast LA, Dupuis P, et al. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC medicine. 2016;14(1):153
The diet-induced increase in testosterone levels was 1.8 nmol/L (52 ng/dL), while the diet + testosterone treatment resulted in an elevation in testosterone levels of 7.4 nmol/L (230 ng/dL). There was no between-group difference in overall adverse events, or serious adverse events. It was concluded that while dieting men receiving placebo lost both fat and lean mass, the weight loss with testosterone treatment was almost exclusively due to loss of body fat.[4]
Symptom response
At baseline, all men reported a moderate degree of symptoms, assessed by the Aging Male Symptoms (AMS) scale, and the international index of erectile function (IIEF-5) questionnaire. It was found that 56 weeks of testosterone treatment improved symptoms of androgen deficiency over and above the effects of weight loss alone.[5]
The initial rapid weight loss was associated with a 20% improvement in total AMS score after the 10-week very-low calorie diet phase, with no difference among participants assigned to testosterone treatment or placebo. This suggests that while symptomatic improvements associated with weight loss may override the effects of testosterone treatment in the short term, testosterone treatment sustains these symptomatic improvements in the longer term.[5]
Compared with placebo, erectile function in men assigned to testosterone was improved in those with baseline erectile dysfunction (baseline IIEF-5 score ⩽20).
Effect on adipokines and gut hormones
Addition of testosterone treatment led to reductions in circulating leptin levels beyond what was achieved by caloric restriction alone.[6] The higher the baseline adiposity, the greater the leptin lowering effect of testosterone compared to placebo treatment. Changes in levels of adiponectin and gut hormones were similar between testosterone and placebo treated men during the study. In both groups, weight loss associated changes in these hormones were evident after the 10 week diet phase, and these changes persisted at week 56 despite 46 weeks of weight maintenance.[6]
Low testosterone levels may contribute to leptin resistance in obese men.[20] Notably, this study suggests that the testosterone effect on reducing circulating leptin – i.e. increasing leptin sensitivity - is not simply an indirect effect mediated by testosterone-associated reductions in body fat.[6] It can be speculated that testosterone treatment may restore HPT axis responsiveness to leptin, which may in turn promote further reduction in fat mass.
Comments
Interestingly, this study is the first testosterone treatment study which was exclusively performed in obese men (BMI ≥30).[4] This study also is one of the few to specifically investigate the effects of testosterone treatment in conjunction to a calorie restricted diet. A previous study found that adding testosterone treatment to a calorie restricted diet + exercise resulted in significantly greater improvements in testosterone levels, HbA1c, fasting plasma glucose, HDL, triglyceride levels, and waist circumference after 52 weeks of treatment, compared with diet + exercise alone.[21] Based on Adult Treatment Panel III guidelines, 81.3% of the patients randomized to diet + exercise + testosterone no longer matched the criteria of the metabolic syndrome, compared to only 31.3% of the diet + exercise alone participants.[21] It was concluded that addition of testosterone to a supervised diet + exercise program results in greater therapeutic improvements of glycemic control and reverses the metabolic syndrome after 52 weeks.[21]
While calorie restriction is the first-line treatment of obesity, its benefits are limited by loss of lean body mass.[12] As lean body mass is associated with a greatly reduced mortality risk, independently of fat mass and cardiovascular and metabolic risk factors [22-25], treatment modalities that preserve lean mass during dieting are needed. As the study presented here shows, testosterone therapy fills this need.[4]
One reason for the lack of preservation of lean mass during the actual diet may be the short diet duration of 10 weeks, as testosterone-induced changes in lean mass typically take several months.[26] Because gains in skeletal muscle mass (lean mass) are correlated with testosterone dose and achieved testosterone levels [27-29], one can also raise the question if it would be possible to entirely prevent the loss of lean mass by achievement of a higher physiological testosterone level? The increase in testosterone levels from a mean baseline level of 8.2 nmol/L (237 ng/dL) to 15.6 nmol/L (450 ng/dL) at study end (week 56) may possibly have been too small to prevent loss of lean mass during the initial calorie restricted diet. Nevertheless, the finding that testosterone therapy attenuates the reduction in lean mass after a diet + maintenance phase should be highlighted, as other treatments that cause large reductions in body weight, such as bariatric surgery [30, 31], also cause large reductions in lean body mass.[32]
It should also be pointed out that the differences in body composition (greater reduction on body fat and preservation of lean mass) occurred despite the modest increase in endogenous total and free testosterone levels (2.9 nmol/L [84 ng/dL] and 30.3 pmol/L [10.5 pg/mL] respectively) with 10.8 % weight loss in placebo-treated men.[4] This diet-induced elevation in testosterone levels is similar to what has been seen in previous weight loss studies [33, 34], and suggests that the endogenous rise in testosterone subsequent to dieting is not sufficient to prevent diet-related loss of lean mass.
The subjects in this study were advised to perform at least 30-minutes of moderate-intensity exercise every day. It is interesting that only men receiving testosterone maintained increased activity levels at study end after 1 year.[4] Previous studies have shown that testosterone therapy consistently improves mood and feelings of energy, and reduces fatigue [35-40]; this in turn may bolster motivation and the ability to adhere to diet and exercise programs.[21, 41, 42]
Summary
Overall, these results indicate that, compared to men receiving placebo who lose both fat mass and muscle (lean) mass during a calorie restricted diet followed by maintenance, adding testosterone treatment to the same diet shifts weight loss to almost exclusive fat mass loss. In addition, testosterone treatment in conjunction with a diet results in greater symptomatic improvements and increased leptin sensitivity, compared to diet alone.
In summary, this new line of research shows that giving testosterone therapy to obese men who are on a diet confers greater improvements in body composition (larger fat loss and reduced loss of lean mass) and symptomatic response than diet alone. These new data also show that diet + testosterone therapy in addition reduces leptin resistance. These data call for consideration of testosterone therapy as an adjunct to available obesity treatments to increase their efficacy and long-term outcomes.
References:
1. Camacho, E.M., et al., Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol, 2013. 168(3): p. 445-55.
2. Rastrelli, G., et al., Development of and Recovery from Secondary Hypogonadism in Aging Men: Prospective Results from the EMAS. J Clin Endocrinol Metab, 2015. 100(8): p. 3172-82.
3. Corona, G., et al., Obesity and late-onset hypogonadism. Mol Cell Endocrinol, 2015. 418 Pt 2: p. 120-33.
4. Ng Tang Fui, M., et al., Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med, 2016. 14(1): p. 153.
5. Ng Tang Fui, M., et al., Symptomatic response to testosterone treatment in dieting obese men with low testosterone levels in a randomized, placebo-controlled clinical trial. Int J Obes (Lond), 2017. 41(3): p. 420-426.
6. Ng Tang Fui, M., R. Hoermann, and M. Grossmann, Effect of testosterone treatment on adipokines and gut hormones in obese men on a hypocaloric diet. J Endocrine Society 2017.
7. Khera, R., et al., Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA, 2016. 315(22): p. 2424-34.
8. Garvey, W.T., et al., American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for Comprehensive Medical Care of Patients with Obesity. Available at https://www.aace.com/files/final-appendix.pdf (accessed December 30, 2016) 2016.
9. Jensen, M.D., et al., 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol, 2014. 63(25 Pt B): p. 2985-3023.
10. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, O.E.P., Expert Panel Report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity (Silver Spring), 2014. 22 Suppl 2: p. S41-410.
11. Padwal, R., et al., Relationship Among Body Fat Percentage, Body Mass Index, and All-Cause Mortality: A Cohort Study. Ann Intern Med, 2016. 164(8): p. 532-41.
12. Weinheimer, E.M., L.P. Sands, and W.W. Campbell, A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev, 2010. 68(7): p. 375-88.
13. Kalyani, R.R., M. Corriere, and L. Ferrucci, Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol, 2014. 2(10): p. 819-29.
14. Anderson, R.N. and B.L. Smith, Deaths: leading causes for 2002. National Vital Statistics reports. . 2005, National Center for Health Statistics, 2005. (No. 17.).
15. Zamora, E., A. Galan, and R. Simo, [Role of myostatin in wasting syndrome associated with chronic diseases]. Med Clin (Barc), 2008. 131(15): p. 585-90.
16. Fearon, K., W.J. Evans, and S.D. Anker, Myopenia-a new universal term for muscle wasting. J Cachex Sarcopenia Muscle, 2011. 2(1): p. 1-3.
17. Tajar, A., et al., Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab, 2010. 95(4): p. 1810-8.
18. Ng Tang Fui, M., et al., Obesity and age as dominant correlates of low testosterone in men irrespective of diabetes status. Andrology, 2013. 1(6): p. 906-12.
19. Corona, G., et al., Testosterone supplementation and body composition: results from a meta-analysis of observational studies. J Endocrinol Invest, 2016. 39(9): p. 967-81.
20. Jockenhovel, F., et al., Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J Clin Endocrinol Metab, 1997. 82(8): p. 2510-3.
21. Heufelder, A.E., et al., Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl, 2009. 30(6): p. 726-33.
22. Srikanthan, P. and A.S. Karlamangla, Muscle mass index as a predictor of longevity in older adults. Am J Med, 2014. 127(6): p. 547-53.
23. Wannamethee, S.G., et al., Decreased muscle mass and increased central adiposity are independently related to mortality in older men. Am J Clin Nutr, 2007. 86(5): p. 1339-46.
24. Bigaard, J., et al., Body fat and fat-free mass and all-cause mortality. Obes Res, 2004. 12(7): p. 1042-9.
25. Chuang, S.Y., et al., Skeletal muscle mass and risk of death in an elderly population. Nutr Metab Cardiovasc Dis, 2014. 24(7): p. 784-91.
26. Saad, F., et al., Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol, 2011. 165(5): p. 675-85.
27. Storer, T.W., et al., Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc, 2008. 56(11): p. 1991-9.
28. Finkelstein, J.S., et al., Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med, 2013. 369(11): p. 1011-22.
29. Bhasin, S., et al., Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab, 2005. 90(2): p. 678-88.
30. Carrasco, F., et al., Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg, 2009. 19(1): p. 41-6.
31. de Aquino, L.A., et al., Bariatric surgery: impact on body composition after Roux-en-Y gastric bypass. Obes Surg, 2012. 22(2): p. 195-200.
32. Cadegiani, F.A., G.C. Diniz, and G. Alves, Aggressive clinical approach to obesity improves metabolic and clinical outcomes and can prevent bariatric surgery: a single center experience. BMC Obes, 2017. 4: p. 9.
33. Niskanen, L., et al., Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab, 2004. 6(3): p. 208-15.
34. Corona, G., et al., Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol, 2013. 168(6): p. 829-43.
35. Amanatkar, H.R., et al., Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry, 2014. 26(1): p. 19-32.
36. Spitzer, M., et al., The effect of testosterone on mood and well-being in men with erectile dysfunction in a randomized, placebo-controlled trial. Andrology, 2013. 1(3): p. 475-82.
37. Wang, C., et al., Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab, 1996. 81(10): p. 3578-83.
38. Jockenhovel, F., et al., Comparison of long-acting testosterone undecanoate formulation versus testosterone enanthate on sexual function and mood in hypogonadal men. Eur J Endocrinol, 2009. 160(5): p. 815-9.
39. Jockenhovel, F., et al., Timetable of effects of testosterone administration to hypogonadal men on variables of sex and mood. Aging Male, 2009. 12(4): p. 113-8.
40. O'Connor, D.B., J. Archer, and F.C. Wu, Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab, 2004. 89(6): p. 2837-45.
41. Saad, F., et al., Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev, 2012. 8(2): p. 131-43.
42. Kalinchenko, S.Y., et al., Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf), 2010. 73(5): p. 602-12.



