Beneficial effects of testosterone therapy in women on menopause symptoms and quality of life

Testosterone levels in women decline steeply with age during the reproductive years; by the time women reach their late 40, their blood testosterone levels are approximately half what they were in their 20s.[1, 2]
Symptoms of androgen deficiency, including a reduced sense of well-being, dysphoric mood (sadness, depression, anxiety, and irritability), fatigue, decreased libido, hot flashes, bone loss, decreased muscle mass and strength, changes in cognition and memory, and insomnia may occur prior to cessation of menses.[3] Pre-menopausal patients frequently report ‘menopausal symptoms’, most of which are not related to estradiol levels.[4]
Adding testosterone to estrogen therapy in postmenopausal women has beneficial cardiovascular effects[5] and also results in meaningful improvements in sexual function in women not taking estrogen.[6]
Testosterone supplementation in both pre- and postmenopausal women has been shown safe, even in higher doses[7, 8], and shown not to affect the menstrual cycle.[9] It is increasingly used as part of postmenopausal HRT (hormone replacement therapy) regimens.[10] Contrary to old beliefs, testosterone can actually protect against breast cancer. It has been shown that addition of testosterone may counteract breast cell proliferation induced by estrogen/progestogen therapy in postmenopausal women.[11-15]
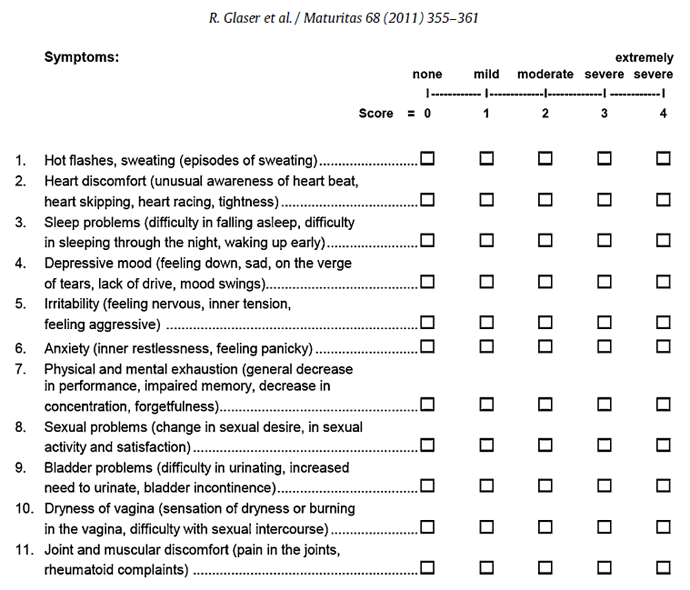
A notable study investigated the effectiveness of a 3 month continuous testosterone therapy, delivered by subcutaneous implant, on the relief of somatic, psychological and urogenital symptoms in both pre- and post-menopausal patients using the self administered Health-Related Quality of Life (HRQOL) questionnaire called the Menopause Rating Scale (MRS).
STUDY DESIGN:
300 pre- and post-menopausal women with symptoms of androgen deficiency, were asked to self-administer the 11-item MRS, at baseline and 3 months after their first insertion of the subcutaneous testosterone implant.
The mean age of the women was 51.7 years.
36.0% were pre-menopausal, 35.3% reported non-surgical, spontaneous menopause (last menstrual cycle greater than 12 months), 19.0% were surgical-menopausal (bilateral oophorectomy with or without hysterectomy), and 9.6% had a hysterectomy with one or both ovaries intact.
Baseline hormone measurements, menopausal status and BMI, were assessed to determine correlation with symptoms and clinical outcome.
TESTOSTERONE THERAPY:
The mean dose of subcutaneous testosterone implanted at the first visit was 121 mg. The range was between 75mg and 160mg. The initial testosterone dose was partially based on weight with a higher dose being used in heavier women. An approximate initial testosterone dose in mg of twice the patients weight in kg has been successfully used in clinical practice.
MAIN OUTCOME MEASUREMENTS:
Changes related to therapy were determined. Total Menopause Rating Scale scores as well as psychological, somatic and urogenital subscale scores were compared prior to therapy and following testosterone implant therapy.
RESULTS:
At baseline before start of testosterone therapy, 87% of the women had free testosterone levels in the lower third of the reference range, 11% of the women had free testosterone in the middle third, and only 2% had free testosterone in the upper third.
There was no difference in distribution of free testosterone between pre- and post- menopausal patients, and there was no significant difference in total testosterone levels between pre- and post-menopausal patients.
Pre-menopausal and post-menopausal females reported similar hormone deficiency symptoms. Both groups demonstrated similar improvement in total score, as well as psychological, somatic and urogenital subscale scores with testosterone therapy. Better effect was noted in women with more severe complaints. Higher doses of testosterone correlated with greater improvement in symptoms.
In all individual Menopause Rating Scale symptom categories (1–11), except dryness of the vagina (10) and anxiety (6), higher doses of testosterone correlated with greater clinical improvement. In addition, after adjusting for dosage based on total body weight, greater improvement in Hot flashes, sweating (1), heart discomfort (2), sleep problems (3), depressive mood (4), physical and mental exhaustion (7), sexual problems (8) and joint and muscular discomfort (11) correlated with higher testosterone dose.
In post-menopausal patients, higher testosterone doses correlated with greater improvement in Menopause Rating Scale total score and all three sub-scores: somatic, psychological and urogenital.
In pre-menopausal patients, higher testosterone doses correlated with greater improvement in Menopause Rating Scale total score and urogenital sub-score.
Over 90% of patients reported less irritability (feeling nervous, inner tension, feeling aggressive) on testosterone therapy, whereas only 4.4% of patients reported a mild increase in these symptoms. This refutes the common concern regarding testosterone's potential to increase aggression and irritability.
Known androgenic side effects include a possible increase in facial hair and mild acne. Some women reported a slight increase in facial hair, but no patient discontinued therapy for that reason. Only three patients discontinued testosterone therapy due to ‘lack of effect’ (which might have been an indication that the dose was too low). No adverse effects were seen on blood sugar, insulin resistance, diabetes, or lipid profiles in follow-up of women for over 2 years.
CONCLUSION:
This study shows for the first time that adequate doses of continuous testosterone alone, delivered by subcutaneous implant, was effective therapy for physical, psychological and urogenital symptoms in both pre- and post-menopausal women. This suggests that testosterone has a physiologic function for women's health. Along with a growing body of research data, this study underscores that testosterone administration improves quality of life in both pre- and postmenopausal women.



